Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kimura, Takaumi; Kirishima, Akira*; Arisaka, Makoto
Kidorui, (47), p.43 - 56, 2005/11
no abstracts in English
Kirishima, Akira; Kimura, Takaumi; Tochiyama, Osamu*; Yoshida, Zenko
Journal of Alloys and Compounds, 374(1-2), p.277 - 282, 2004/07
Times Cited Count:27 Percentile:75.6(Chemistry, Physical)no abstracts in English
Okamoto, Yasuharu*; Mochizuki, Yuji*; Tsushima, Satoru*
Chemical Physics Letters, 373(1-2), p.213 - 217, 2003/05
Times Cited Count:8 Percentile:24.96(Chemistry, Physical)no abstracts in English
Kimura, Takaumi; Nagaishi, Ryuji; Ozaki, Takuo; Arisaka, Makoto*; Yoshida, Zenko
Journal of Nuclear Science and Technology, 39(Suppl.3), p.233 - 239, 2002/11
no abstracts in English
Sugo, Yumi; Sasaki, Yuji; Tachimori, Shoichi
Radiochimica Acta, 90(3), p.161 - 165, 2002/04
Times Cited Count:153 Percentile:99.24(Chemistry, Inorganic & Nuclear)no abstracts in English
Fukumoto, Masahiro; Nishikawa, Yoshiaki*
JNC TN8400 2001-017, 355 Pages, 2001/03
The alkali hydrolysis experiments which seem to be important from the view point of the alteration mechanism using the following seven organic materials was performed as a part of the evaluation of the influence on the disposal of the organic materials contained in the TRU wastes. As a result of the alkali hydrolysis experiments (90 C and 91d), each organic materials became those of lower molecular weight. The degradation products were able to be detected in the solution. The organic materials seem to be degraded to the organic matters which were confirmed in this study in a long term of disposal. The degradation products were shown below. Therefore, the evaluation of the influence on the migration of radionuclides by degradation products becomes important in the future. (1)Cement additives of Naphthalenesulfonic acid and Ligninsulfonic acid (
C and 91d), each organic materials became those of lower molecular weight. The degradation products were able to be detected in the solution. The organic materials seem to be degraded to the organic matters which were confirmed in this study in a long term of disposal. The degradation products were shown below. Therefore, the evaluation of the influence on the migration of radionuclides by degradation products becomes important in the future. (1)Cement additives of Naphthalenesulfonic acid and Ligninsulfonic acid ( Naphthalenedisulfonic acid etc.) (2)Cement additives of polycarboxylic acid (
Naphthalenedisulfonic acid etc.) (2)Cement additives of polycarboxylic acid ( Oligomer of distal methoxypoly ethylene glycol.) (3)Ethylenediamine-N,N,N',N'-tetraacetic acid disodium salt (
Oligomer of distal methoxypoly ethylene glycol.) (3)Ethylenediamine-N,N,N',N'-tetraacetic acid disodium salt ( Acetic acid desorped and cyclized organic matters from EDTA.) (4)Tributyl phosphate (
Acetic acid desorped and cyclized organic matters from EDTA.) (4)Tributyl phosphate ( Dibutyl phthalate, n-butanol) (5)Poly vinyl acetate (
Dibutyl phthalate, n-butanol) (5)Poly vinyl acetate ( Acetic acid) (6)Nylon66 (
Acetic acid) (6)Nylon66 ( Adipic acid, Hexamethylenediamine) (7)Cured epoxy resin (
Adipic acid, Hexamethylenediamine) (7)Cured epoxy resin ( Glycerol poly glycidyl ether, Carboxylic acid)
Glycerol poly glycidyl ether, Carboxylic acid)
Makuuchi, Keizo; Haque, M. H.*; Ikeda, Kenichi*; Yoshii, Fumio; Kume, Tamikazu
Nihon Ratekkusu Arerugi Kenkyukai Kaishi, 4(1), p.7 - 10, 2001/01
no abstracts in English
Moriyama, Hirotake*
JNC TJ8400 2000-050, 47 Pages, 2000/03
In support of the safety assessment of geologic disposal of high levcl radioactive wastes, the solubility of transuranium elements was studied. The solubility of PuO
 xH
xH O was measured undcr a reducing condition, and the solubility product K
O was measured undcr a reducing condition, and the solubility product K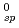 and the stability constant
and the stability constant 
 of Pu(OH)
of Pu(OH) were obtained. The obtained K
were obtained. The obtained K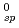 value was found to be much smaller than that predicted by Rai et al. from its dependence on ionic radius. Also, the solubility of PuO
value was found to be much smaller than that predicted by Rai et al. from its dependence on ionic radius. Also, the solubility of PuO 3
3  xH
xH O was measured under an oxidizing condition and the solubility product K
O was measured under an oxidizing condition and the solubility product K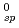 was obtained. In the analysis of hydrolysis constants of actinide ions, it was found that the systematic trend of the hydrolysis constants was well explained by the hard sphere model considering the effective charges of actinide ions.
was obtained. In the analysis of hydrolysis constants of actinide ions, it was found that the systematic trend of the hydrolysis constants was well explained by the hard sphere model considering the effective charges of actinide ions.
Br chle, W.*; Eichler, B.*; G
chle, W.*; Eichler, B.*; G ggeler, H. W.*; Guenther, R.*; J
ggeler, H. W.*; Guenther, R.*; J ger, E.*; Jost, D.*; Kratz, J. V.*; Nagame, Yuichiro; Paulus, W.*; Pershina, V.*; et al.
ger, E.*; Jost, D.*; Kratz, J. V.*; Nagame, Yuichiro; Paulus, W.*; Pershina, V.*; et al.
Proceedings of 1st International Conference on the Chemistry and Physics of the Transactinide Elements, 3 Pages, 1999/00
no abstracts in English
Oda, Chie; *
JNC TN8400 98-001, 14 Pages, 1998/11
Solubilities of amorphous stannic oxide, SnO (am) in Na-ClO
(am) in Na-ClO -Cl-SO
-Cl-SO aqueous systems were measured to quantitatively investigate the influences of the ligands OH
aqueous systems were measured to quantitatively investigate the influences of the ligands OH , Cl
, Cl and SO
and SO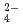 on solubilities. They were also measured in bentonite equilibrated solutions to discuss the behavior of tin under a repository condition of a high-revel radioactive waste. The solubility data in sodium perchlorate media in the range of pH from 6 to 11 showed pH dependency, and the hydrolysis constants of tin (IV) were determined (Amaya, et al., 1997). No significant changes in solubilities with the variation in Cl
on solubilities. They were also measured in bentonite equilibrated solutions to discuss the behavior of tin under a repository condition of a high-revel radioactive waste. The solubility data in sodium perchlorate media in the range of pH from 6 to 11 showed pH dependency, and the hydrolysis constants of tin (IV) were determined (Amaya, et al., 1997). No significant changes in solubilities with the variation in Cl , SO
, SO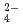 concentrations were observed in Na-ClO
concentrations were observed in Na-ClO -Cl-SO
-Cl-SO aqueous systems, so this indicates that chloride and sulfate species were less effective than hydroxide complexes. On the other hand, solubilities in bentonite equilibrated solutions were higher than solubilities of other experiments in simple systems. These results suggest that (1) other complexes of tin except hydroxide, chloride or sulfate complexes of tin (IV) may dominantly exist in aqueous phase, (2)solid phase other than SnO
aqueous systems, so this indicates that chloride and sulfate species were less effective than hydroxide complexes. On the other hand, solubilities in bentonite equilibrated solutions were higher than solubilities of other experiments in simple systems. These results suggest that (1) other complexes of tin except hydroxide, chloride or sulfate complexes of tin (IV) may dominantly exist in aqueous phase, (2)solid phase other than SnO (am) may limit the solubility of tin under repository conditions.
(am) may limit the solubility of tin under repository conditions.
M.Schaedel*; W.Bruechle*; E.Jaeger*; B.Schausten*; G.Wirth*; W.Paulus*; R.Guenther*; K.Eberhardt*; J.V.Kratz*; Seibert, A.*; et al.
Radiochimica Acta, 83(3), p.163 - 165, 1998/00
no abstracts in English
G.Meinrath*; Kato, Yoshiharu; Kimura, Takaumi; Yoshida, Zenko
Radiochimica Acta, 82, p.115 - 120, 1998/00
no abstracts in English
Kato, Yoshiharu; Kimura, Takaumi; Yoshida, Zenko; Shirasu, Noriko
Radiochimica Acta, 82, p.63 - 68, 1998/00
no abstracts in English
Niimura, Nobuo
Seibutsu Butsuri, 38(4), p.167 - 169, 1998/00
no abstracts in English
Hidaka, Akihide; Igarashi, Minoru*; Hashimoto, Kazuichiro; *; Sugimoto, Jun
Journal of Nuclear Materials, 248, p.226 - 232, 1997/00
Times Cited Count:1 Percentile:14.48(Materials Science, Multidisciplinary)no abstracts in English
 Co adsorbed on sand
Co adsorbed on sandTanaka, Tadao; Muraoka, Susumu
Radioisotopes, 45(12), p.753 - 760, 1996/12
no abstracts in English
Hidaka, Akihide; Igarashi, Minoru*; Hashimoto, Kazuichiro; *; Sugimoto, Jun
UTNL-R-0337, 0, p.35 - 46, 1996/00
no abstracts in English
Kimura, Takaumi; ; G.Meinrath*; Yoshida, Zenko; Choppin, G. R.*
JAERI-Conf 95-005, Vol.2, 0, p.473 - 485, 1995/03
no abstracts in English
Tamada, Masao; Asano, Masaharu; Spohr, R.*; Vetter, J.*; Trautmann, C.*; Yoshida, Masaru; Katakai, Ryoichi*; Omichi, Hideki
Macromolecular Rapid Communications, 16, p.47 - 51, 1995/00
Times Cited Count:8 Percentile:42.89(Polymer Science)no abstracts in English